Information for Surgical Trainees
The Sunflower study – funded by the NIHR HTA – is the UK’s largest RCT investigating the management of patients with gallstone disease. It aims to compare the clinical and cost-effectiveness of expectant management and MRCP prior to laparoscopic cholecystectomy and will recruit 7,457* patients from more than 50 UK hospitals. The contributions of trainees are therefore vital, and we are very much looking forward to working with you!
*this target was reduced in an approved amendment in 2023


Click here for: Frequently Asked Questions for Surgical Trainees
Recent work by the Trainee Executive Group:
Please find attached the latest Trainee Newsletter Jan 2024 .

Our previous Trainee Newsletters:
~~~
Please find below a set of slides which can be used as the basis for trainee induction in the study:
Generic Site Junior Doctor Induction Sunflower ppx (1)
Please also find here a Top Tips document that is great document to have at your site. It covers all sorts of key points around recruitment including flagging up new trainees to the study, hpw to advertise the study and how to explore different routes of recruitment e.g emergency and elective pathways.

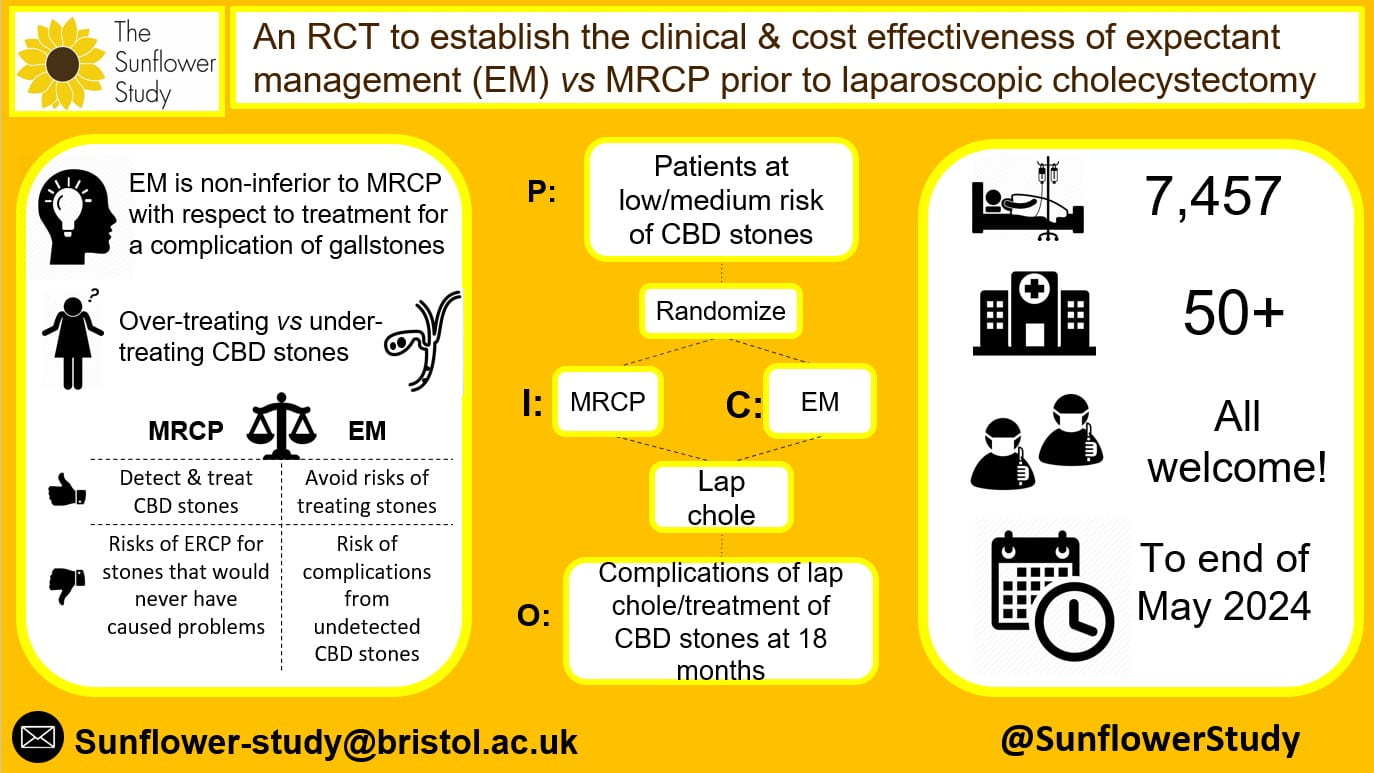
Infographic showing the main features of the study
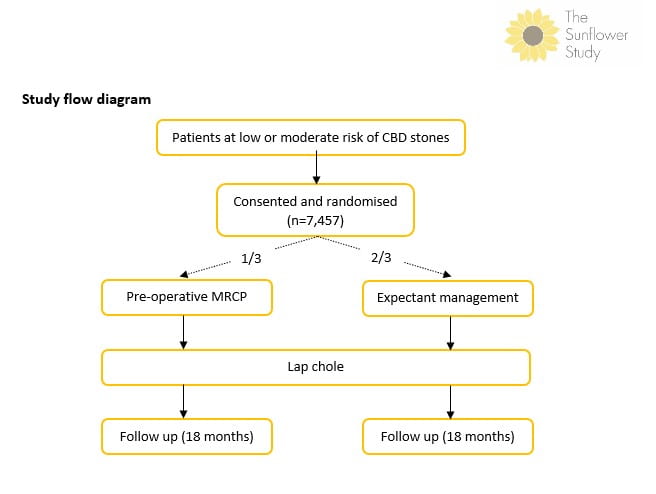
Study flow diagram
Trainee benefits of being involved in the Sunflower study
Research logbook: We have worked hard to ensure that all of your efforts are recognised: screening and identifying eligible patients, recruitment consultations, collecting baseline or follow up data, and co-ordinating trainees’ efforts on a local or regional level. Much like an operative logbook, we will be able to provide you with a breakdown of your activities at regular intervals, to demonstrate your contributions for the purposes of ARCPs, interviews, portfolios etc.
CCT requirements: Participating in the Sunflower study will provide you with the opportunity to complete various CCT requirements, including: i) GCP training, ii) leadership role, iii) recruitment of patients to a national study, iv) research methods training. We will also provide you with training in how to best recruit patients into an RCT, see FAQs below.
NIHR Associate Principal Investigator (PI) Scheme
We fully endorse this exciting new scheme run by the NIHR. We aim to have two associate PIs in every recruiting centre.
The scheme helps junior doctors to be PIs of the future. It aims to engage, recognise and promote junior doctor engagement in NIHR portfolio research. It was developed by the West Midlands Research Collaborative, Birmingham Surgical Trials Consortium, Birmingham Clinical Trials Unit and the West Midlands NIHR Clinical Research Network.
The Associate PI scheme has been endorsed by the NIHR Clinical Research Network’s (CRN) Cancer, Surgery and Oral & Dental Specialty Cluster and the Royal College of Surgeons (England).
There can be two Associate PI per site, per study, at any given time. A commitment of at least 6 months will be required for gaining Associate PI status, after which the NIHR CRN will issue a certificate to confirm Associate PI status.
Please email sunflower-study@bristol.ac.uk if you would like to be considered for this prestigious role. If you have any queries about the scheme, you can contact associatepischeme@nihr.ac.uk or find further information on the scheme at the NIHR website.
Trainee roles and responsibilities in the Sunflower study
Regional lead
Manage day to day queries from local leads and collaborators within their geographic region
Disseminate information about the study to participating centres in their geographic region
Help to recruit local leads and collaborators
Present the study at regional educational and research meetings
Liaise with the central Sunflower team to update on progress and problems
If their hospital is participating in the study:
Identify and recruit patients
Contribute to completion of case report forms and follow up data
Work within the local research team (consultants, trainees, nurses)
Local lead
Act as an official Associate PI, within the scheme endorsed by the NIHR (see separate documentation)
Work with the consultant PI to lead the project in a single institution
Recruit and manage local collaborators
Present the study at local departmental meetings
Identify and recruit patients
Contribute to completion of case report forms and follow up data
Work within the local research team (consultants, trainees, nurses)
Local collaborator
Identify and recruit patients
Contribute to completion of case report forms and follow up data
Work within the local research team (consultants, trainees, nurses)
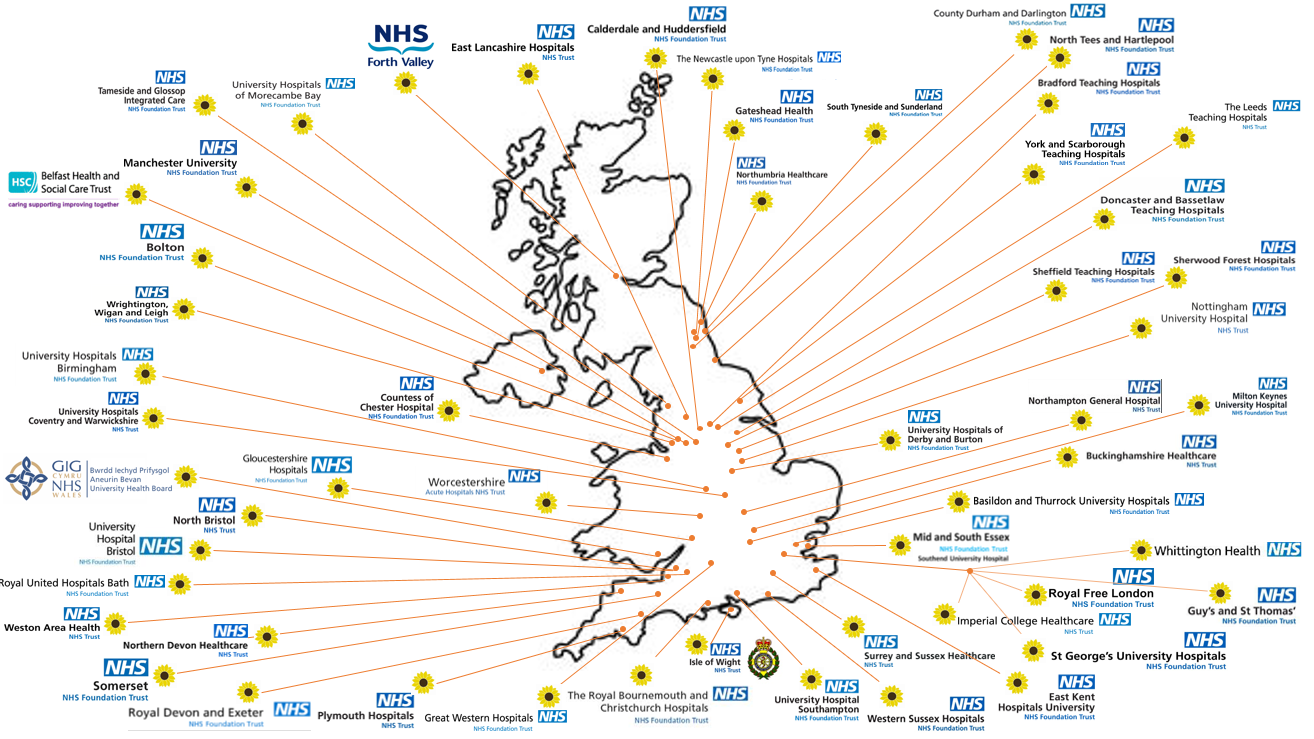
Map of participating sites – as of 19/01/2022
Recruitment Flow Diagram