The Sunflower Study: A report from the 4th Investigators Meeting
Over 90 representatives from 38 study sites attended our 4th in-person Sunflower investigators’ meeting in Birmingham on Monday 14th March. There were a series of talks from the study team and from representatives from recruiting centres, and a strong focus on audience participation with challenges aired and many solutions shared. Initial feedback is that the meeting was very well received, and we hope that the study will continue to recruit well. In this report, we provide an overview of the content of the talks, and some pictures from the day. We hope these are useful to those who could not attend, and also to those who were there.
Welcome and study update Giles Toogood and Marcus Jepson

Giles thanked the attendees for their commitment to the study & gave an overview of the study purpose, and progress. There are now 55 Sunflower sites with more in the pipeline. Looking at predicted against actual number of patients recruited, prior to the COVID-enforced pause the two lines were running in parallel. Since sites re-opened from June 2020, the lines had diverged and (to use a cricketing metaphor) we are now behind the required run-rate. Pre-COVID 200-300 patients were randomised per month. Since re-opening, that figure is slowly increasing to around 100 per month. The target, however is 388 per month, so we anticipate a need to extend the recruitment period by 18-24 months (although this is unconfirmed). Giles reported that time to surgery post randomisation had changed from median of 122 days (pre-pause) to 246 days (post pause). This represents the reduced amount of operating in hospitals during the pandemic. The proportion of urgent patients recruited has increased post-pause, and Giles welcomed this change. He also pointed out that many of the centres near the top of the league table were those with an ambulatory care service. Finally, Marcus encouraged those who want acknowledgement on study papers to ensure that their GMC/NMC number is entered onto the database.
Threats and opportunities for Sunflower posed by the COVID-19 pandemic Barney Reeves, Bristol Trials Centre

Barney gave an overview of the impact of Covid-19 on Sunflower. He explained that the proportion of participants who are not having lap chole has almost doubled from 22% to 36% since the re-start. The time from randomisation to lap chole has almost doubled from a mean of 115 to 220 days.
He looked at the pros and cons of the study continuing unchanged. The C-GALL found no difference in quality of life for patients with uncomplicated symptomatic gallstones, whether or not they underwent lap chole. However, the Sunflower research question is different and still important – our patient group and outcomes are different, so Sunflower represents a huge research learning experience for sites and trainees. Nevertheless, there is a need for a costed extension and it is unclear how rapidly clinical activity, research capacity and recruitment rate can be restored.
Barney asked the attendees to consider whether the primary outcome timing should be extended beyond 18 months after randomisation, as operations are being delayed. Other areas also need attention, such as the impact of a drop in hospital admissions overall – will the study be underpowered? Are there more crossovers? However, some opportunities have arisen from COVID. The delay to lap chole provides more variation in primary outcomes and allows modelling of the influence of such a delay.
Update on other relevant research Jane Blazeby

In this section, Professor Blazeby gave the meeting an update on other research relevant to Sunflower. She outlined a systematic review recently undertaken of published and ongoing RCTs and observational studies looking at outcomes for patients with symptomatic gallstone disease given lap chole plus a common bile duct stone diagnostic procedure prior to, or during, surgery. The review, carried out by Katy Chalmers, Sian Cousins, Natalie Blencowe, analysed studies from January 2013 to December 2021. Five studies were included, four observational and one randomised. Results were deemed to be non-conclusive and the reviewers felt that the Sunflower study is still needed in order to discover the importance of MRCP in reducing complications of lap chole.
Keynote Address: RCS England Research Initiatives: past, present and future Prof Peter Hutchinson RCS Trials initiative

Professor Peter Hutchinson, Director of Clinical Research at the Royal College of Surgeons of England, gave the meeting an overview of surgical research in his keynote address.
From the early days of case studies to modern randomised controlled trials, surgical research has expanded and developed over its history. Prof Hutchinson says that a large proportion of the surgical literature is of questionable value, so ongoing efforts are made to improve its quality.
Since 2011, we have seen exponential growth in NIHR portfolio trials with an expertise in delivering high quality surgical research, involving consultants, trainees, academic and NHS staff including statisticians, clinical trial units, and health economists.
Prof Hutchinson highlighted the role of the collaborative model of authorship, and the work of regional Surgical Research Collaboratives to support research activities among rotating trainees. Each region now also has a Clinical Research Network Specialty Lead in Surgery.
The RCS Research Fellowship scheme has awarded 850 fellowships over its 27 years, offering paid time out of clinical environments to answer research questions of importance to surgery. Many other grants and fellowships are awarded, such as Pump Priming Grants for newly-appointed consultants, and the NIHR Health Technology Assessment Program to fund research on new NHS treatments.
The RCS COVID Research Group undertook more than 50 studies to aid policy and practice during the pandemic, working internationally with adult and paediatric patients.
Prof Hutchinson believes the future of surgical research depends on supporting trainees and consultants to carry out more commissioned trials, particularly on the rapid advances in digital technology that will allow surgery to become safer and less invasive.
A successful team approach Richard Young, Zoe Scott and Harriet Pearson (York & Scarborough)

The Trust carries out about 650 Lap Choles annually, with around 30% of patients undergoing MRCP. A close relationship with the radiology team has contributed to the site’s recruitment success, with both a surgical and radiology API in place. As a PI, Richard benefits from local expertise at this highly research active Trust, as well as valuable expertise from working in Leeds. He also pointed to the helpful NIHR courses and the use of technology to enhance teamworking including the Pando App which is approved for sharing confidential data. The team from York & Scarborough (Richard Young, Sunflower PI & Research Nurses Zoe Scott and Harriet Pearson) gave a description of the local work. This is Richard’s first study as a PI although he has long been involved in research. He emphasised the importance of teamwork and sharing insights and experiences within the team.
Trainees are key, he said, to identify patients and as a focal point of contact for the wider team. Having a radiology API is a great way to promote the study in the radiology department.
Crucial to their local success is having a clear checklist for any team member who contacts the patient, and significant help from admin support staff. But improvements can still be made, Richard explained, in clinician recruitment of acute patients, better explanation of completing the consent form, and greater efforts to keep reinforcing the study to clinicians.
Steering a steady ship through the pandemic Owain Jones and Nichola Kearsley (Chester)

PI Owain Jones and Research Nurse Nichola Kearsley at the Countess of Chester Hospital talked us through their Sunflower experience.
They carry out about 300 lap choles annually, but have a current waiting list of over 200 patients. There is an established nursing research team but with no background in upper GI. Their ‘plan of attack’ for the study was to gain buy-in from all biliary consultants, get an aPI in place, search outpatient clinic lists and the waiting list. In year 1 all went well except a lack of buy-in from consultant colleagues and a low consent rate versus total number of operations. March 2020 was their best month to date. Following the re-start, a large proportion of patients on the waiting list are now ineligible due to having MRCP, and it is becoming clear that the face-to-face recruitment yield is higher than by telephone or post.
Research staff have returned to Sunflower study and clinical practice remains below pre-pandemic levels but is increasing quickly. Key to their progress has been a team of 11 or more staff working together on consenting, paperwork, and a local Whatsapp group, as well as highlighting the study at local meetings, study days and hospital inductions. Further success has come from screening clinic lists in advance for eligible patients, and the site has maintained its place in the top few recruiting centres for several months.
PI experiences of centre challenges Liz Gemmill (Kings Mill)

Liz Gemmill (PI) told the meeting that Kings Mill Hospital is a 600 bed district general hospital in North Nottinghamshire, with four upper GI consultants. The site has seen a number of personnel changes since recruiting to Sunflower, including several APIs.
Issues faced by the site – apart from the obvious challenges of the pandemic – include a problem convincing consultant colleagues to take part in recruitment, and a change of clinics to majority telephone appointments. However, Miss Gemmill and the Kings Mill team have identified a number of possible solutions including having more conversations with colleagues to raise enthusiasm about the study, offering to help colleagues with study tasks, regular reviews of Patient Tracking Lists (PTLs) and a return to face to face clinics. The current API is highly research-driven, Miss Gemmill added, and she anticipates this will help to bolster their recruitment.
Why the Sunflower study needs trainees, and why trainees need the study Jane Collingwood (Trainee Coordinator) Kat Butcher (a newly qualified consultant at Weston)

Around 300 trainees are contributing towards Sunflower, and over 100 have now registered onto the NIHR Associate PI scheme via the study. They reminded the meeting attendees that trainee involvement in the study is a win-win, benefitting the study, site PIs and the trainees themselves. These advantages are described in Marcus Jepson’s qualitative paper which can be viewed here: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-021-05536-7
Of particular importance is the role of trainees in recruiting out-of-hours and acute patients, whose data is crucial for answering the study question, and who are essential to reaching the overall recruitment target. There are several misunderstandings about the API scheme so please do contact Jane (jane.collingwood@bristol.ac.uk) at any stage of the process for guidance, and remember, radiology trainees can now join and will become valuable members of the local Sunflower team.
MRCP qualitative data and perspectives Ashley Guthrie (Leeds)

Dr Guthrie, a Sunflower co-applicant based in Leeds, gave the meeting an overview of the qualitative radiology data and his perspectives on the study so far. A third of Sunflower patients randomised to pre-operative MRCP will follow their hospital’s usual radiology protocols, with imaging taking place at an outpatient or inpatient appointment, depending on initial presentation.
To address the challenge of MRCP technique varying across centres, images and reports for a random 10% of patients having MRCP are sent by electronic image transfer to radiologists in Leeds for review.
The MRCP technique will be evaluated by looking at the number and type of pulse sequences used. Quality is measured by subjective visual assessment and concordance with hospital reports. This provides an insight into current NHS practice and variation in MRCP technique within the study.
Ashley told us that local protocols on MRCP technique vary between sites, for example some use combinations of different types of 2D T2 weighted images with or without fat saturation and others 3D. Variation in slice thickness is one of the other major imaging parameters.
Ashley presented an outline of a review of the Leeds MRCP protocol. The longstanding “traditional” protocol for the identification of ductal stones based on two types of 2D sequences was evaluated and audited against ERCP. One of the 2D options was then replaced with a 3D sequence and the process repeated. A protocol based on axial and coronal 3 mm 2D (FISP) images was found to maintain sensitivity and the other diagnostic sequence dropped resulting in significantly shorter examination times (reduced from approximately 21 mins to 10 mins). Ashley encourages radiologists at other centres to look critically at their imaging protocols.
Sunflower – a patient perspective Kerry Avery and Marcus Jepson, University of Bristol & on behalf of Liz Booth, PPI lead
Kerry and Marcus have interviewed Sunflower patients about their experiences of taking part in the study. Most patients approached for Sunflower have consented (c75%), but still over 1000 patients have declined, so they looked at interviews to explore whether any aspects of the study approach or information could be improved. The most common response patients gave for taking part in Sunflower was that they felt happy to help with research, but importantly also because they knew they would be well looked after if they took part in the study. We suggested it was important to impress this second point when discussing Sunflower with patients.
Some patients felt the timing of when they were approached for Sunflower wasn’t ideal – in each case they said they were in pain and this had impacted on their decision making. In the open floor discussion one site stated that they always made sure patients pain relief was sorted out before speaking to them about Sunflower. Patients had been asked to say what they remembered Sunflower being about, and there were mixed levels of recall and understanding (ranging from the very accurate: ‘to find out the dis/advantages of having an MRI before GB surgery’ to quote one patient, to the quite inaccurate ‘it’s study to decide whether or not gallbladders should be removed‘.
The audience agreed that it was important for patients to be aware of the purpose of Sunflower.
Why are we collecting cost and Quality of Life data? Will Hollingworth

Will discussed the importance of sites getting high completion rates on the patient questions collecting information about patient quality of life and time off work. These questionnaires are collected in a selection of sites which is rotated throughout the trial. Pre-pandemic completion rates were high (>80%), but have fallen post-pandemic (57%) with quite wide variation between sites in completion rates.
Currently the following sites should be collecting questionnaire data from all patients randomised.
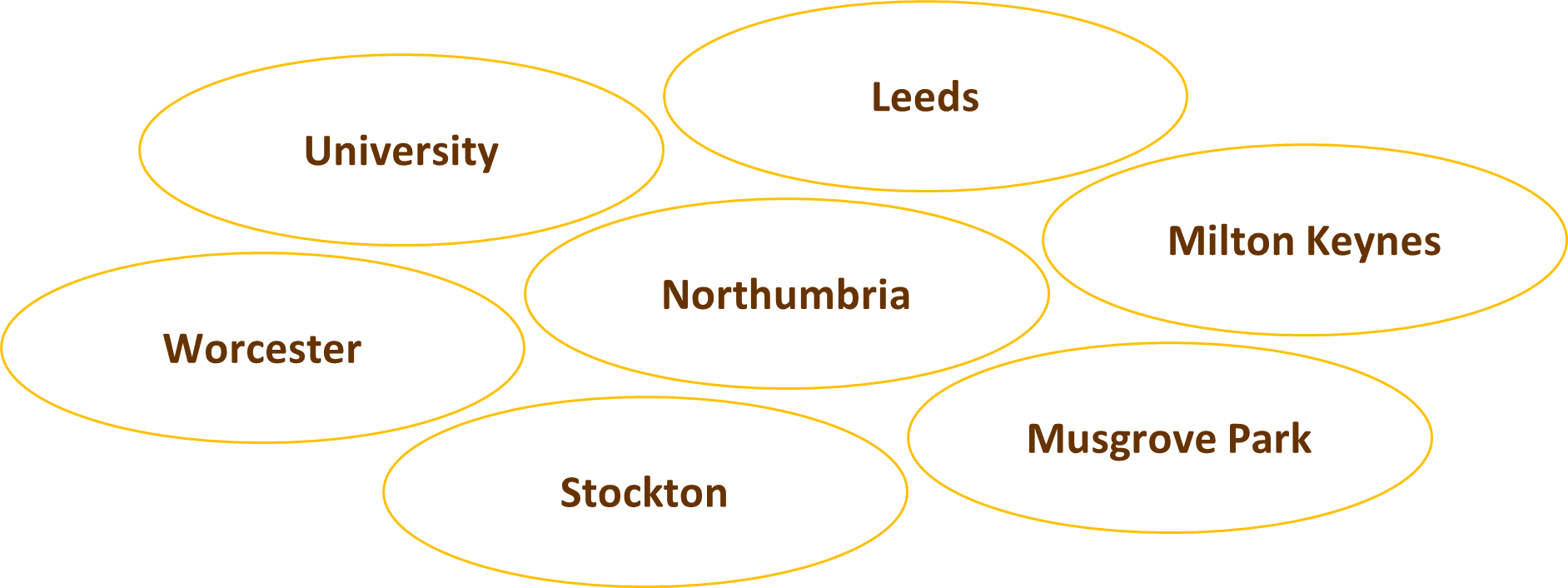
Baseline questionnaires should be completed before randomisation alongside the consent form or when completing the baseline details in the CRF. In the original protocol, the Sunflower Study also asked for sites to collect the questionnaire at the time of the Index Admission for Laparoscopic Cholecystectomy. But we want to clarify that this requirement has been temporarily suspended (to ease site burden during the pandemic recovery) and is not currently required. All subsequent questionnaires (at 3 months, etc.) are managed centrally by the Bristol team. Please ensure patient contact details are correct. Thank you so much and please do keep completing baseline questionnaires with all of your new randomised patients.

