Information for NHS Sites
Recruitment for the ‘Sunflower Study’ has now been completed, and we are no longer accepting new participants. This page is being maintained for reference purposes only. Thank you to all the hospitals, surgeons, , nurses, radiologists, surgical trainees, patients, amongst others who have contributed to the study. Your involvement has been invaluable to the progress of this research. For any further information regarding the study or its findings, please refer to the links provided or contact the study team directly.
What is the purpose of the study?
At the moment, it is not known whether patients at low/medium risk of common bile duct stones should be investigated with an MRCP prior to their gallbladder surgery – fewer than 10% of these patients will have common bile duct stones.
The Sunflower Study will provide information to optimise treatment benefits and minimise the harms in patients awaiting gallbladder surgery who are at low/medium risk of common bile duct stones. The study will provide an estimate of the risk of complications of gallstones (in the gallbladder or common bile duct) for at least 12 months after gallbladder surgery. The study will also estimate the cost effectiveness of MRCP compared to no MRCP (expectant management).
Some common bile duct stones pass spontaneously before or after gallbladder surgery. Estimates of the spontaneous passage of common bile duct stones are difficult to obtain and this study will be able to provide these. It is thought that, for low/medium risk patients, up to 75% of common bile duct stones may pass spontaneously
How can we become a participating site?
The Sunflower Study will run in at least 50 NHS Hospital Trusts in England, Wales, Scotland and Northern Ireland. All sites will require access to MRI facilities.
If you would like to participate in the study, please email sunflower-study@bristol.ac.uk and provide:
Name and email address of a local PI
Names and email addresses of local participating surgeons
Name and email address of a local R&D contact
Name and email address of a local radiologist supportive of the study
Will there be excess treatment costs?
The Sunflower Study involves randomising participants in a 1:2 ratio to MRCP:expectant management.
The level of excess treatment costs will depend on your current level of MRCP – it may be less than 1 in 3, roughtly 1 in 3 or more than 1 in 3.
The study will be providing some site payments, and is on the NIHR Portfolio.
What is the recruitment target for my site?
The Sunflower Study is looking for sites to recruit around 40% of their eligible patient population.
The exact recruitment target will be determined as part of the feasibility process.
Can surgical trainees get involved in the study?
The Sunflower Study is very keen for trainees to be involved in patient consent and data collection for the study. We are also keen to involve trainees in the NIHR Associate PI scheme.
Please contact the Sunflower team for more information – sunflower-study@bristol.ac.uk
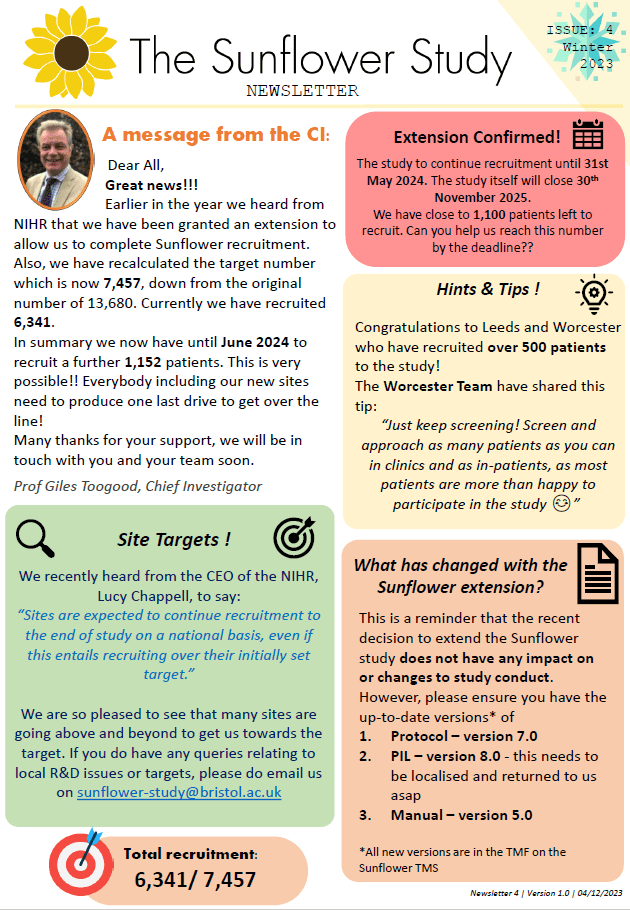
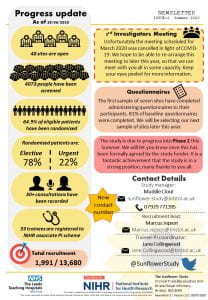
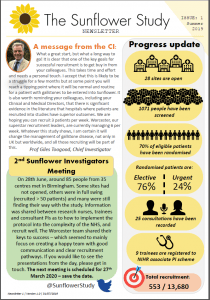
The total recruitment target for the study is 7,457* patients over a recruitment period ending in June 2024.
*this target was reduced in an approved amendment in 2023
What is the radiology involvement in the study?
The Sunflower Study is looking at the use of MRCP in gallbladder surgery patients. The study will make no changes to routine MRCP protocols.
We are collecting information on the types of scanners used at sites and their standard imaging protocols.
The data collection forms for the study will collect information on the results of any MRCPs carried out on study participants.
We will also be asking for sites to provide a 10% sample of their MRCP images and reports for Quality Assurance review, using the Image Exchange Portal service.
Study Management System
The Sunflower study management system is web-based and provides a link to the patient database.
You will need to register for an account to access this system. Please contact the Sunflower study team for registration instructions (sunflower-study@bristol.ac.uk).
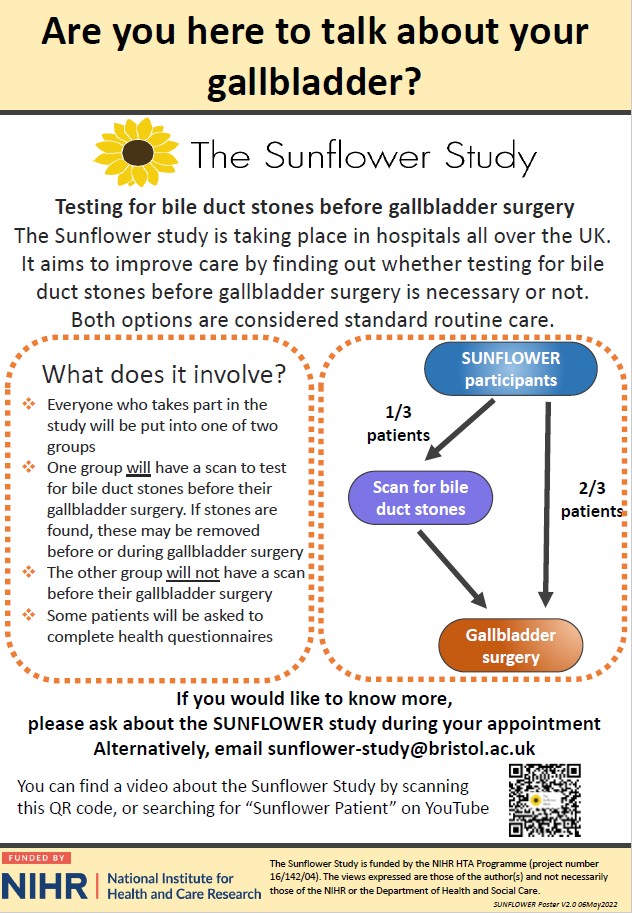
New Patient-facing Poster v2.0
On 9th May 2022 we were approved to use a new version of our patient-facing poster. Our patient and participant involvement (PPI) group have helped to hone this poster. We hope that the addition of a QR code may help patients learn more about the study and potentially encourage participation.
Site newsletters
Below is our most recent newsletter shared with site staff in spring 2024: Click here for a downloadable version: Sunflower_Newsletter_5_Spring 2024 v1.0